Filtros IVC
Índice
ToggleRetirada del mercado del filtro VCI y lesiones
Información y qué hacer
Un filtro de vena cava inferior, o Filtro IVCes un dispositivo implantado para impedir que los coágulos sanguíneos lleguen a los pulmones del paciente. Por desgracia, los filtros VCI mal diseñados y defectuosos han causado daños irreparables a miles de pacientes. Las lesiones más comunes incluyen la perforación de órganos/venas, la migración del filtro desde el lugar del implante y la creación de coágulos sanguíneos.
Los pacientes lesionados y sus familiares pueden tener derecho a una indemnización por daños y perjuicios a través de una demanda por un filtro IVC. La mayoría de las demandas por responsabilidad civil de productos IVC se están consolidando en expedientes de tribunales federales diseñados específicamente para abordar las lesiones generalizadas causadas por filtros defectuosos. Debido a que hay miles de víctimas del filtro IVC con diferentes lesiones, es importante que documente su daño ampliamente y busque un recurso legal rápidamente con la ayuda de un abogado de lesiones por filtro IVC. Además de las demandas por filtros IVC, muchos pacientes que han sufrido a causa de dispositivos médicos defectuosos también buscan justicia a través de Demandas por mallas de hernia. Si usted o un ser querido ha sufrido complicaciones a causa de implantes de mallas para hernias, también puede tener derecho a una indemnización.
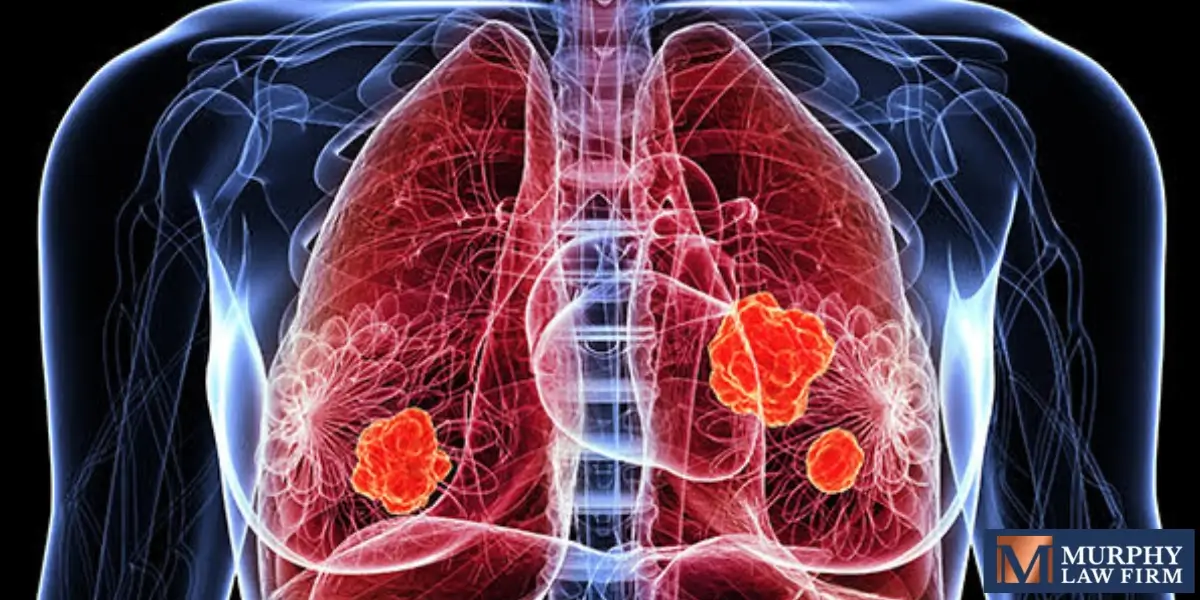
Peligros de los filtros VCI
La finalidad de los filtros de vena cava inferior es impedir que los coágulos sanguíneos lleguen a los pulmones, una afección potencialmente mortal conocida como embolia pulmonar (EP). Sin embargo, muchos fabricantes de filtros VCI no diseñaron ni fabricaron correctamente estos dispositivos. Según un estudio médico, el 40% de los filtros VCI implantados se habían desplazado de su posición óptima.
El tipo de defecto y daño causado varía entre los pacientes, pero las lesiones y problemas más comunes de la VCI incluyen:
- Perforación o punción de órganos y vasos sanguíneos
- No filtra los coágulos sanguíneos dañinos después de que el dispositivo migre o se incline
- Daños o muerte por fracturas del filtro VCI, que envía fragmentos a través del torrente sanguíneo.
- Coágulos de sangre, o embolización, causados por el filtro VCI
- Trombosis venosa profunda (TVP) de miembros inferiores
Los filtros VCI se diseñaron para utilizarse poco tiempo -la Administración de Alimentos y Medicamentos de EE.UU. (FDA) indica que el tiempo ideal de implantación es de 29 a 54 días-, pero muchos dispositivos permanecen en el paciente más tiempo o fallan antes de poder retirarse. Además, la extracción del filtro intravenoso suele ser molesta y presenta sus propios riesgos.
Advertencias del Gobierno
En 2004, el fabricante de dispositivos médicos C.R. Bard contrató a un consultor de litigios para que estudiara la peligrosidad de sus filtros IVC de nuevo diseño frente a los modelos más antiguos. El informe, conocido como Informe Lehmann, se elaboró únicamente con fines internos y se filtró accidentalmente. Su admisibilidad como prueba en las demandas por responsabilidad civil por el uso de filtros IVC es muy controvertida. Como mínimo, el informe indica que C.R. Bard sabía que sus filtros IVC presentaban efectos secundarios graves para los pacientes, pero no compartió ese conocimiento con los pacientes ni con la FDA.
En 2013, un estudio publicado en el Journal of the American Medical Association (JAMA) examinó los riesgos y beneficios relacionados con la duración del implante del filtro VCI de un paciente. Tras examinar los historiales médicos disponibles, los investigadores descubrieron que muchos médicos no retiraban a tiempo los filtros VCI a sus pacientes cuando ya había pasado la amenaza de embolia pulmonar.
Los científicos revisaron los datos disponibles para hallar un plazo ideal para retirar los dispositivos de filtrado de VCI a los pacientes, y llegaron a la conclusión de que entre 29 y 54 días después de la implantación suele ser el óptimo. Los filtros intravenosos presentan una elevada tasa de fallos que aumenta cuanto más tiempo permanece implantado el dispositivo. La investigación, descrita como puntuación neta de riesgo, reveló que el riesgo de acontecimientos adversos empezaba a aumentar entre cinco y ocho semanas después de que a un paciente se le implantara un filtro VCI.
Modelos y fabricantes de filtros IVC
Los fabricantes y modelos responsables de la mayoría de las lesiones por filtros IVC en EE.UU. incluyen:
- C.R Bard
- Filtro Bard G2
- Filtro de recuperación Bard
- Filtro Bard G2 Express
- Cook Medical
- Cocinero Celect
- Tulipán Gunther
El filtro IVC específico responsable de sus problemas de salud puede influir en el lugar donde debe presentar su demanda. Obtenga más información en la sección siguiente.

Litigios sobre filtros IVC
El número de pacientes lesionados por filtros IVC está creciendo rápidamente, por lo que los tribunales estadounidenses están organizando las demandas contra Cook Medical en un proceso conocido como litigio multidistrito (MDL). Las demandas por filtros IVC contra Cook Medical se están consolidando en el Tribunal de Distrito de EE.UU. en el Distrito Sur de Indiana.
Las demandas contra C.R. Bard aún no se han consolidado, pero es probable que eso ocurra pronto. Los fabricantes demandados suelen estar a favor de las MDL porque les permiten defender -o resolver- sistemáticamente los pleitos en un único tribunal, ante un único juez y en una única instancia. Baton Rouge.
En algunos casos, la demanda de una víctima de lesiones no es apropiada para su consolidación en el expediente de la MDL. En abogado experto en productos sanitarios defectuosos puede ayudarle a decidir qué enfoque es el mejor para su demanda de filtro IVC.
Obtenga ayuda con su reclamación por lesiones del filtro IVC
Murphy Law Firm, LLC está representando a las víctimas del filtro IVC y aceptando más clientes. Nuestro bufete de abogados ha representado con éxito a miles de personas con reclamaciones de responsabilidad por productos que involucran dispositivos médicos defectuosos o medicamentos farmacéuticos. Nuestro equipo de abogados en Murphy Law Firm tiene la experiencia que su reclamación necesita para conseguirle una indemnización completa.
Para una consulta inicial GRATUITA con un abogado fundador Peyton P. Murphyllame al (225) 928-8800o contacto en línea.

Su camino hacia la resolución legal comienza con el bufete de abogados Murphy.
Cuando usted elige Murphy Law Firm, usted obtiene acceso a un equipo de abogados experimentados con un historial probado de éxito. Entendemos la importancia de la confianza, y nos esforzamos por construir relaciones duraderas con nuestros clientes mediante la entrega de resultados excepcionales.

Llame para una consulta
Áreas de práctica
- Demandas colectivas
- Demandas colectivas
- Indemnización por accidente laboral
- Accidente por exceso de velocidad
- Accidente con fuga
- Accidente en una intersección
- Accidente T-Bone
- Colisión frontal
- Colisión trasera
- Accidente de scooter
- Accidente de vehículo comercial
- Accidente de autobús
- Accidente de peatón
- Lesiones catastróficas
- Lesión por parálisis
- Lesión medular
- Daño cerebral traumático
- Lesión de rodilla
- Lesión de hombro
- Lesión cervical
- Lesión de espalda
- Accidentes de bicicleta
- Accidentes de tráfico
- Accidentes de camiones
- Accidentes de vehículos todoterreno y ATV
- Accidentes de tráfico
- Accidentes de autocaravanas y remolques
- Accidentes de autobús escolar
- Accidentes ferroviarios
- Accidentes marítimos
- Muerte por negligencia
- Accidentes por resbalones y caídas
- Mordeduras de perro
- Negligencias médicas
- Accidentes de moto
- Accidentes por conducir ebrio
- Reclamaciones al seguro de coche
- Accidentes por conducción distraída
- Accidentes de motoristas sin seguro y con seguro insuficiente
- Accidentes por vuelco
- Accidentes de camión relacionados con el alcohol y las drogas
- Accidentes por negligencia del conductor de camión
- Accidentes de camiones sobrecargados
- Accidentes por fatiga del conductor de camión
- Accidentes con camiones defectuosos
- Accidentes de barco
- Reclamaciones salariales
- Accidentes de barcos de alquiler
- Accidentes de cruceros
- Lesiones del marinero de cubierta
- Accidentes de barcos pesqueros
- Lesiones de trabajadores de transbordadores
- Ley Jones
- Ley de limitación de responsabilidad
- Accidentes en alta mar
- Lesiones en plataformas petrolíferas
- Negativa a pagar gastos médicos
Testimonios
¡Peyton siempre está ahí cuando lo necesitas!
-Danyielle Whatley
Estoy orgulloso de haber elegido a Murphy Law Firm para manejar mi caso.
-Arthur Davis
Si alguna vez necesito ayuda, ¡me aseguraré de llamar al bufete Murphy!
-Fallon Carter
¡¡¡¡¡Un servicio increíble!!!!! ¡¡¡¡¡Yo recomendaría a todo el mundo a Murphy Law Firm!!!!!
-Austin Stinson
¡¡¡Gran abogado!!! ¡Muy amable y bien informado!
-erin guidry
¡¡¡¡Me impresionó mucho!!!! Siempre me llamaban el mismo día.
-erin guidry
¡Peyton ama a los niños! ¡Un hombre increíble!
-Chad Wilson
Muchas gracias, chicos. Seguid trabajando así de bien.
-Jonell Foster
Es la mejor empresa con la que he hecho negocios.
-Anthony Jones
Excelentes servicios y recomendaría a otros para negocios.
-Deshanna Martin
Definitivamente recomiendo Peyton y su bufete de abogados.
-Dan Clan
Muy satisfecho con el resultado y con la puntualidad con la que se resolvió.
-Jody miller
¡Gran trabajo! Os recomendaría a cualquiera.
-Michelle Williams
No sea víctima dos veces, este bufete de abogados hará el trabajo. Están del lado de la víctima, hasta que ganan.
-Steve Miller
Peyton Murphy y su equipo son los mejores.
-BRsocial Life
Estaba experimentando lo amo el personal impresionante y muy servicial
-chosenchild2013
Trabajó muy duro para resolver mi caso, hizo un gran trabajo.
-Frank Robinson
Sin lugar a dudas, Peyton Murphy tiene el mejor bufete de abogados en Baton Rouge.
-JBeat_iT
Gracias por ayudar a nuestra comunidad. El campamento de fútbol es una experiencia maravillosa.
-Cjs22
Me escucharon y me prepararon con gran esmero.
-Heather Gettle
Me encanta como me cuidaron...y se aseguraron de que me fuera con una sonrisa 😃
-Marlisha Roberts
Aunque estamos atravesando una pandemia, fueron muy profesionales y atentos durante todo el proceso.
-Sharon Adams
"No te conformes con Centavos". Estoy muerto. jajajaja
-Conrad Green
Se los recomendaría a todo el mundo. El Sr. Brian fue genial.
-Makayla Gross
Aconsejaré a cualquiera que utilice a Peyton. Es más que un abogado, es un verdadero amigo...
-charmaine james
Peyton y Troy realmente trabajaron para darme lo que merecía.
-Tia Fulghum
¡Jo y Jim hicieron un gran trabajo! Gran servicio.
-Katreena Cavin
Trabajan muy duro. Están muy orientados a la familia y tienen el máximo interés en hacerlo lo mejor posible.
-Deseada Annette Lovins (Melocotones)
¡¡¡Siempre cumple, justo cuando lo necesito!!! 👍🙂
-Reg D.
¡Me encanta mi abogado! Mantenga el gran trabajo Sr. Murphy.
-Coop Coop
Jim y Joelene cuidaron muy bien de mí durante todo el caso.
-Alexus Winston
Servicio impresionante, me sentí como si yo fuera su único cliente. Comprensión precisa y fácil de mi caso.
-Rebecca Johnson
Jim y Jolene hicieron un trabajo maravilloso durante todo el proceso del caso.
-Tracy Johnson
La Mejor Experiencia!✨✨✨✨✨💐 Muy Agradable!!! ¡¡¡Y el Doggy🐕 TOPPED IT!!! Lol Dulce
-Kristy Mays
Gracias a Murphy Law Firm, mi mamá recibió un gran acuerdo y pude ir a una escuela privada ... ¡¡¡GRACIAS PEYTON!!!
-Kamar Meza
Muy profesional. Manejó mi caso de manera excelente. Recomiendo encarecidamente Peyton.
-Brett Ransome
Muy amable y simpático, estoy satisfecho con mis visitas y mi caso.
-Anova Null
¡Vayan con el bufete Murphy! ¡Son geniales! Mi caso se resolvió muy rápidamente.
-Courtney Perry
Proceso amistoso y rápido. Jim y Jo son muy educados y profesionales también.
-Aaliyah Robertson
Me puse en contacto con Murphy Law Firm después de mi accidente de motocicleta y el Sr. Morain me recibió con los brazos abiertos, incluso pagó por mi cirugía.
-Michael Harris- Bey
Realmente no pensé que este caso pasaría pero gracias a Murphy Law Firm. Muy respetuoso e incluso vinieron un par de horas en un domingo a mi casa.
-Heather Manuel
Troy con el bufete de abogados de Peyton Murphy fue muy atento a mi caso. ¡Yo recomendaría este bufete de abogados a todo el mundo!
-Tamara Jones
¡Servicio impresionante de la mejor firma de abogados en la ciudad! Fue un placer trabajar con Jim y Jo.
-Jason Faul
Se toman su tiempo para entender los problemas y trabajan contigo. Me mantuvieron informado en todo momento y escucharon mis preocupaciones.
-Alex N
¡El bufete de abogados Murphy es como una familia! ¡Ellos resolvieron este caso y se aseguraron de que yo estaba satisfecho!
-ROBIN MILLIGAN
¡Son los mejores y trabajan muy rápido! ¡Los recomiendo encarecidamente!
-Clorissa Dunn
Brian era increíble, un gran tipo con el que trabajar y un equipo increíble.
-devin penalber
Ha sido una experiencia muy sencilla y profesional. Todo el mundo ha sido muy amable y profesional.
-Josie Adams
Muy profesional y las manos en todo el proceso. ¡Yo recomendaría Murphy Law Firm a cualquiera que necesite un abogado de lesiones personales!
-Sheila Murphy
Nos ayudaron mucho con nuestros casos. Si no los hubiéramos tenido, creo que nos habrían presionado y nos habrían pagado menos de lo que merecíamos.
-Brandon Adkins
Llevo 20 años tratando con Peyton, es como de la familia.
-Jenean Mitchell
Ustedes son los mejores. Peyton & todos trabajaron diligentemente con el fin de resolver mi reclamo. Grandes corazones perfectos personalmente y fue un honor tener a ustedes como mi equipo #WhoaNation💛💜💛💜🦁 #TigerNation🦁💛💜🦁💛💜🦁 Gracias Murphy Law Firm🦁🦁🦁🦁🦁.
-gorrión damón
Mi marido, David Legnon, agradece todo lo que han hecho por él. Gracias a todos por todo.
-Belinda Legnon
Esta es la tercera vez que utilizo la experiencia de este bufete de abogados y cada vez los resultados superaron las expectativas. Muchas gracias a Peyton, Troy y equipo.
-Lawrencia Gougisha
Llame a Peyton Murphy cuando necesite un abogado de lesiones personales. Él realmente se preocupa por sus clientes y luchará contra la compañía de seguros para obtener la compensación que se merece.
-Ron Richmond
Personal muy amable, el personal es tranquilizador y te explica todo de principio a fin. Llaman a menudo sobre el progreso de su caso y manejar cualquier preocupación que pueda tener.
-Leo Gaines III
El bufete de abogados Murphy siempre me ha dado un gran servicio. Si le dedicas tiempo, te dará grandes resultados. Seguiré recurriendo a él cuando lo necesite. 👍🏾
-kevin bova
Mi abogada la Sra. Candy me ayudó en un mes. ¡Todo fue perfecto! Estas personas son una bendición.
-Tremiah W
Al principio era muy escéptico sobre mi caso, pero ha sido la mejor experiencia que he tenido nunca. Ni siquiera esperaba el resultado. Gracias Murphy Law Firm.
-Andrea Akakpo
Mi nombre es Tyrone G. Soy cliente de Peyton Murphy. Su servicio fue rápido y profesional y quedé muy satisfecho. ¡Muchas Gracias!
-Verde TIRONE
Gran empresa, buen servicio al cliente, muy buenos resultados 😍😍😍 Especial Sra. Jolene y Sr. Jim, están dispuestos a ayudarme siempre que lo necesito.
-John Le
El Sr. Murphy tiene una personalidad impresionante y su personal se comunica muy bien. Sin duda recomendaría su servicio.
-Sempleadora Stringer
Gran empresa, gran gente. Se encargaron de todo lo que necesitaba y más. Realmente aprecio Peyton y Jolene. Yo recomendaría a cualquiera.
-Shaunice M
Peyton se atiene a su lema. Nunca serás una víctima dos veces. Gracias por cuidar tan bien mi caso y el de mi nieta.
-Patsy Jackson
Jo fue muy servicial, la recomendaría en cualquier momento. ¡Realmente me gusta trabajar con ella! ¡Murphy Law Firm es el lugar!
-Ruby aikens
Hola! Yo recomendaría y recomendaré el bufete de abogados Murphy. Son los mejores y él tiene los pies en la tierra y me encanta su gran bebé (perro). Son rápidos y se mueven rápido. Gracias Murphy Law Firm
-El Club Fwum
Murphy es el mejor - estado tratando con él más de 30 años y siempre te dan la 💵💰
-dipper joseph
Sr. Jim Moore y Jo eran grandes. Me ayudaron en cada paso del camino. Era tan fácil. Recomiendo verdad el bufete de abogados de Peyton Murphy.
-Jason Buckley
¡Muchas gracias a Jordania y Jolene por trabajar tan duro en mi caso! ¡Aprecio todo de Murphy Law Firm !
-Elizabeth Mcgehee
Jordan y Jo me ayudaron mucho. Un gran equipo de personas... ¡GRACIAS Peyton!
-Wesley Daigle
Peyton y Troy parecen muy preocupados por conseguir lo máximo para sus clientes. ¡Cuando usted tiene preocupaciones, asegúrese de hacerles saber y van a manejar la situación!
-Raven Viada
Estoy muy satisfecho con Murphy Law Firm. Yo recomendaría a cualquiera que busque asesoramiento jurídico y la acción de venir a ver al Sr. Peyton Murphy y Troy Moran.
-James McClay
Quiero dar las gracias al Sr. Brian por un trabajo bien hecho. Si pudiera, le daría un 10. Fue un placer trabajar con usted. Fueron dos largos años, pero le doy la gloria a Dios.
-Denise Wilson
Murphy Law Firm y Jolene y Jim siempre me ayudó y salió de su manera de conseguir mi dinero. Me encanta todo el mundo allí ❤️
-Diana Medina
Mi experiencia con Murphy Law Firm fue genial. El personal era tan dulce y agradable, me ayudaron a través de mi naufragio y eran tan útiles también. Fue una gran experiencia. ¡¡¡¡¡¡Yo los usaría de nuevo!!!!!!
-Timothy Mcmanus
Una empresa que se mantiene en contacto contigo para darte actualizaciones o simplemente para saber que no están sentados sin hacer nada. Normalmente es el cliente el que llama para hacer tantas preguntas como si funcionara como las palomitas de maíz. Todas las cosas llevan su tiempo. Prefiero un mensaje de texto o un correo electrónico a llamar y sentirme impaciente.
-Charisse Harris
¡Murphy Law Firm es amable y profesional! Jim y Jo me han guiado a través de este proceso con facilidad. Recomiendo encarecidamente Murphy Law Firm.
-Bobbi Golden
Hicieron un gran trabajo llevando mi caso. Todos respondieron a mis preguntas cuando las tuve, y me aseguraron que estaban trabajando para mí. Gracias Murphy Law Firm.
-Erika Keller
¡Sobresaliente! ¡Peyton y su personal fue más allá con un esfuerzo súper humano para ayudarme, incluso cuando hay muy poco dinero para hacer, Peyton mantuvo su palabra y siguió luchando por mí!
-David Vizinat
Peyton Murphy y asociados son abogados atentos y profesionales. Me sentí cómodo que estaba en buenas manos. Ellos me cuidaron.
-Corol Perry
Todos fueron muy amables y comunicativos. Me trataron como si fuera de la familia.
-Toby
Sr. Jim y Jolene me hizo sentir cómodo y respondió a cualquier pregunta que tenía con mi caso, yo estaba muy satisfecho. ¡¡¡Si usted está en necesidad de un abogado, sin duda recomiendo Murphy Law firm!!!
-Denisha Knox
Mi nombre es Kasha Dugas, yo era un cliente de Murphy Law Firm. ¡Tuve un asistente legal increíble Sra. Jo! ¡¡¡Y el Sr. Jim y el Sr. Peyton fueron a batear para mí, muy increíble !!!
-todd perez
La mejor experiencia. Ellos no te dejan en la oscuridad acerca de su caso. El Sr. Murphy me llamó y personalmente repasó mi caso. Definitivamente los recomiendo.
-Josh Daigrepont
¡Servicio A1! ¡El bufete de abogados Murphy son el número 1 en mi libro! Jo y Jim son los asistentes perfectos de Peyton.
-Kenneth Peltier
Peyton Murphy es el mejor abogado en Baton Rouge. Él se preocupa por sus clientes y te hace sentir como en familia. Yo recomendaría el bufete de abogados Murphy a todo el mundo.
-Josh Guidry
El bufete de abogados Murphy me ha representado durante 20 años. Jim y Jo han trabajado duro y hacer el trabajo. Gracias. - Kendra Jackson
-Xclusive Ryderz
Murphy Law Firm se aseguró de que todo para mí fue atendido desde el inicio del proceso hasta el final. ¡Yo recomendaría sus servicios a cualquiera!
-Leigh Blanchard
Peyton Murphy es uno de los mejores abogados que conozco en Baton Rouge, Louisiana. Es muy informativo y conmovedor, considera las opiniones de sus clientes y es muy sensible a las necesidades y deseos del cliente. Es un buen hombre de familia y, sobre todo, se preocupa por la comunidad y sus clientes. Yo recomendaría Peyton Murphy ...
-Leah Brown
Murphy Law Firm me ofreció el mejor servicio después de mi accidente de coche. Todos sus empleados son super amables y serviciales. ¡100% recomiendo!
-Lanie
¡Me encanta Peyton y Denise! Se tomaron su tiempo y me explicaron todo en detalle. ¡Mi caso fue mucho tiempo y lo hicieron el trabajo! ¡Gracias Murphy Law Firm #1 en mi libro!
-Erica Antoine
Fueron muy amables y se portaron muy bien conmigo. Se los recomendaría a todo el mundo. Todo el personal era encantador. Realmente se trata de ti allí y me encanta eso. Gracias Murphy Law Firm
-Sabrina Gilliland
El bufete de abogados Murphy es el mejor. Mi familia y yo sabemos que siempre podemos contar con ellos. Ellos van más allá y realmente los apreciamos. Nosotros 1000% recomendamos Murphy Law Firm.
-Cindy W.
Este es el mejor bufete de abogados en Baton Rouge, LA. ¡¡¡La Sra. Candy es INCREIBLE!!! Ellos salen de su camino y realmente se preocupan por usted como persona. Recomiendo a todos y cada uno a Murphy Law Firm.
-Dawn Krys Thibodeaux
He tenido una experiencia excelente con el bufete Murphy. Muchas gracias a Jim y Jo Estoy muy emocionado con la cantidad que me devolvieron.
-Michelle Shavers
Brian y Peyton fueron excelentes. Siempre me mantuvieron informado sobre el proceso y se aseguró de que tenía el mejor resultado. Se los recomiendo a cualquiera que necesite ayuda legal.
-Zana williams
Tanto Jim como Jo fueron de gran ayuda durante todo el proceso y siempre estuvieron disponibles para ayudarme con cualquier duda que pudiera tener. ¡Un gran servicio al cliente!
-Azere C
¡Murphy Law Firm Literalmente puedo escribir un libro! He estado bajo esta firma durante casi 25 años que son los mejores que hacer el trabajo que tienen la espalda siempre que son más que un bufete de abogados para mí somos familia nunca un momento aburrido siempre preguntando ¿necesitas algo ...
-Melinda Watkins
Excelentes abogados. ¡Personable, hablar con usted acerca de cualquier cosa que necesite, y le ayudará tanto como sea posible! ¡Troy y Jen son increíbles en lo que hacen! ¡Gracias a todos por todo lo que hacen y han hecho por mí!
-Scott Michael
La señora Jolene es la mejor. No sólo llamé a esta señora como cada dos días cada semana. Ella respondió a mi llamada. Recomendaría altamente allí servicio a cualquier persona. Ustedes son los mejores
-Shay Barnes
¡Peyton Murphy fue un excelente abogado! Él me mantuvo al día con los detalles de mi caso e hizo toda la prueba fácil. Definitivamente voy a recomendar Murphy Law Firm.
-Denise Robertson
Me ayudaron como nunca me habían ayudado. Fueron pacientes y me mantuvieron al tanto de todo. Si alguien tiene alguna demanda, recomiendo ir a Murphy Law Firm.
-Delanor Palmer
Yo totalicé mi coche en i12, fui apresurado a la sala de emergencia y recibí una tonelada de cuentas médicas. Obtuve Murphy Law Firm, me ayudaron con las facturas médicas, un coche de alquiler durante 30 días o más, y con la obtención de un fisioterapeuta para recuperarse de mis lesiones. Ellos son todo sobre...
-Tu'Kira Gibson
Quería dar las gracias a Jim y Jo y los chicos de la firma de abogados Murphy por toda su ayuda durante este proceso con este accidente de coche. Ellos ayudaron a volver a una pronta recuperación ❤️❤️ .
-Donovan Mckee
Me refirieron a Murphy Law Firm, y no podría estar más satisfecho. El personal era increíblemente agradable y fácil de trabajar. Mi caso se resolvió bastante rápido y me mantuvieron informado a medida que avanzaba.
-corey williams
Estaba muy contento con el servicio que recibí de Murphy Law Firm. El proceso de pagar por este servicio fue perfecto para mi cartera. Al tener un ingreso fijo, nunca pensé que podría pagar un abogado, pero con esta firma pude hacerlo. Mi experiencia fue increíble. ¡Pruebenlos!
-Connie Rider
El Sr. Murphy fue muy profesional y agradable. Él no se especializa en el área de la ley que mi problema está en. Sin embargo, fue muy útil y sin duda volvería si alguna vez necesito un abogado en su especialidad.
-Lisa Snowden
Peyton Murphy y todo su personal realmente escucharon lo que yo quería y no me presionaron para "ir por el oro". Necesitaba meses de terapia física. Tengo todo lo que necesitaba y se hizo "todo" de nuevo. No me hice "rico" pero volví a tener buena salud sin necesitar 6 cifras para hacer...
-Diane Foster
Muy profesional y el cuidado de sus clientes. Usted puede confiar en que la gente está trabajando para usted y usted no puede ir mal con Murphy Law Firm.
-Connie Dubois
Extremadamente satisfecho de haber elegido Peyton y su personal para manejar mis casos en los últimos 20 años. El tiempo vale la pena y lo más importante es que las habilidades de comunicación son perfectas 💯 Gracias Peyton y personal para un trabajo bien hecho 😉 Dios bendiga.
-Natasha Bova
Después de tener mi accidente, no estaba seguro de qué hacer. Llamé a Murphy Law Firm para obtener más información y al instante supe que quería que me ayudaran con mi caso. Peyton Murphy me aseguró que obtendría lo que merecía del accidente. Peyton y su equipo fueron tan...
-Jadonna Alemania
Me puse en contacto con la MLF al día siguiente de mi grave accidente de moto, cuando aún estaba en el hospital. Desde el primer día, no he recibido nada más que respeto, ayuda y trabajo sincero hecho para mí a expensas del bufete de abogados. Peyton Murphy salió de su bolsillo para un montón de cosas debido a mis circunstancias ...
-jessie lafferty
Sinceramente el bufete Murphy me sacó de un gran apuro. Había una anciana que estaba en necesidad de máscaras y cuando llamé tuve la bendición de hablar con Jennifer que juntos compartimos nuestras historias acerca de nuestra pasión por las personas sin hogar y fue realmente increíble lo mucho que ella y...
-Lady JAGUAR
¡¡¡¡¡La Ley de Murphy es la mejor!!!!! Personal y servicio excepcional. Siempre capaz de hacer preguntas. Murphy ha sido un amigo durante 20 años. Gracias a usted ya su equipo por todo el trabajo duro.
-Fredia Johnson
Murphy Law Firm manejó mi caso de lesiones personales y tengo que decir que estaba muy satisfecho. Sr. Murphy y su personal eran muy conocedores, profsssional y servicial. La mejor parte fue que eran muy justos con los honorarios del abogado. MEJOR ABOGADO DE LESIONES PERSONALES en Baton Rouge manos hacia abajo.
-Mike Simmons
Recomiendo encarecidamente este bufete de abogados. Son muy profesionales, amables, trabajadores, y sin duda hacer el trabajo. Estoy muy satisfecho con el resultado. Si usted está buscando un gran abogado para que lo represente, Peyton en Murphy Law Firm es el mejor.
-Tresha B
¡Esta firma es realmente de primera clase! ¡Han ido más allá y he tenido una gran experiencia con los abogados y el personal de esta empresa! Se los recomendaría a cualquiera que busque abogados brillantes.
-Patrick Glenn
Mi caso fue manejado perfectamente. Todo lo que cabría esperar, que recibí, y la señora Jen era profesional, educado, y con los pies en la tierra y respondió a cualquier pregunta que había hecho. Recomiendo 10 de 10 cualquier día de la semana. Gracias Murphy Law Firm
-Brandon Cormier
El accidente en el que estuve me costó 2 años y medio de dolor, terapia médica y física. State Farm no quería pagarme lo que me debían. Peyton, Troy y su personal consiguieron que me pagaran lo que me debían. Realmente fue un proceso que pasar, pero no podía pedir nada mejor. Murphy...
-Steven Boyer
Profesionalidad total viene a la mente inmediatamente cuando escucho el nombre de "Peyton Murphy" y su equipo de profesionales. Desde el primer día Peyton y su equipo estuvieron ahí para satisfacer cualquier necesidad que tuviera. Y cualquier pregunta que necesitaba respuesta. De tomar la conjetura de averiguar qué médico podría satisfacer mejor mis necesidades. Como...
-Sean Turner
Troy Morain en Peyton Murphy Law Firm o cualquiera de sus abogados para el caso es la persona que desea que le guía a través de este proceso. Siempre me mantuvieron al día y el resultado después de todo fue un jonrón. Recomiendo encarecidamente el bufete de abogados Murphy.
-Zach Moore
Llevo más de 20 años tratando con esta oficina. Nunca he tenido ningún problema. Siempre me han dado el mejor asesoramiento. Incluso si quería hacer ciertas cosas me llamaban para explicarme las circunstancias de lo que podía pasar. Nunca fue nada oculto. He recomendado a mucha gente....
-anyetta woods
Murphy Law Firm es un bufete de abogados de confianza y me gustaría recomendar a mi familia y friends.I 'he aprendido que Mr.Peyton Murphy tiene un corazón cariñoso para las comunidades y sus clientes y para mí que hablan volume.Thank Sr. Peyton Murphy y gracias Mr.Brian por su amabilidad y asegurarse de que lo...
-Jeanella Sanders
Murphy Law Firm fue muy profesional en tomar mi caso. La Sra. Joe era agradable y profesional cuando hablaba con ella sobre el caso con lo que estaba pasando. Aprecié su servicio.
-Ronderick Banks
Murphy Law Firm fue la mejor decisión que tomé después de entrar en un accidente de coche. Peyton Murphy es el mejor abogado en Baton Rouge. Luchó por mi caso y me ayudó a obtener la compensación que merecía. Tuve dolor de espalda y cuello después de mi accidente, y lo último que quería era el estrés ...
-Denise Bonnette
Yo estaba muy contento con la forma en que Murphy Law Firm manejó mi caso. El coche que me golpeó tenía una compañía de seguros que no quiso trabajar conmigo. Me puse en contacto con Murphy Law Firm y fueron muy eficientes en poner todo en orden. Muy educados, profesionales y oportunos.
-Tracy Byers
El bufete de abogados Murphy es un bufete absolutamente fantástico que convirtió una circunstancia muy pegajosa e intensa en un feliz para siempre. No tenía ni idea de cómo resolver un asunto, el bufete Murphy entró y al final de la experiencia mi prometida salió muy agradecida y satisfecha.
-Dewayne Tibbs
Mi suegro me recomendó este bufete de abogados después de mi accidente. Debo decir que me dirigió en el camino correcto. Murphy era impresionante. El bufete de abogados me dio la bienvenida con los brazos abiertos en mi primera visita. Mi caso un caso muy difícil, pero debo decir que tiene el trabajo hecho ....
-Skip Me
Murphy Law Firm 10/10 muy recomendable. Brian fue genial y me mantuvo informado durante todo el proceso. ¡Fue muy amable y bien informado!
-Tiana Young
¡De principio a fin Peyton Murphy y asociados han ido por encima y más allá! Ellos respondieron a todas las preguntas que tenía y me mantuvo informado con cada detalle de mi caso. ¡Me referiré amigos y familiares a Peyton Murphy y será un cliente para siempre!
-kiko W.
Peyton ha sido mi abogado por más de 12 años. Cada vez que tengo un problema y necesito un abogado siempre puedo llamar a Murphy Law Firm y si no pueden manejar el caso me pueden referir a alguien que pueda. Murphy Law Firm siempre ha cuidado bien de mí. Con esta ley ...
-LADY LG
Murphy Law Firm es el mejor y nunca he tenido ningún problema con ellos. ¡¡¡Jolene siempre responde a mis preguntas cada vez que la llamo, fue un placer trabajar con ella!!! ¡Recomiendo totalmente Murphy Law Firm a mi familia y amigos!
-Kennetta Johnson
Mi nombre es Christine Dileo. He utilizado Murphy Law Firm después de mi accidente y yo estaba muy satisfecho con los servicios. Jim y Jolene fueron excelentes para trabajar. Recomiendo encarecidamente el uso de Murphy Law Firm si está buscando un abogado.
-Amy Dileo
Trabajé con Jim y Joe y el proceso fue largo pero valió la pena. Ellos no se dieron por vencidos en mí y salí con un montón de dinero. Muchas veces pensé que mi caso no llegaría hasta el final, pero la lucha que pusieron para mí lo hizo mucho en...
-Noranda Brown
Bueno, todo lo que puedo decir es que mi abogado y su asistente hicieron todo lo posible para conseguirme todo el dinero que pudieron. Incluso hicieron reducciones en su toma para asegurarse de que me dieron más dinero. Yo estaba muy contento con Peyton Murphy Law Firm y utilizarlos de nuevo.
-David Macaluso
¡Mi experiencia con Murphy Law Firm fue un éxito total! Yo estaba involucrado en un accidente de coche malo en I-12 y mi coche fue total. Peyton mismo llamó para comprobar en mí periódicamente a través de todo el proceso. Aunque debo admitir que él era difícil de alcanzar a veces, él era muy...
-Terrell McGill
Excelente personal, compasivo y muy bien informado en lo que respecta a la ley. Estoy contento con el resultado de mi caso y con Murphy Law Firm. Me alegro de haberlos elegido para que me represente y yo los usaría de nuevo si es necesario. Definitivamente los recomiendo a cualquiera que los necesite.
-Kimberly Polito
Todo el personal es increíble y especialmente mi abogado, Jordania, que estaba ansioso por ayudarme desde el momento después de mi accidente. ¡¡¡Elegiría el bufete de abogados Murphy sobre cualquier bufete de abogados!!! ¡¡¡Muchas gracias al mejor personal!!!
-Believe Spa
Joe, Jordan, y Peyton trabajaron muy duro en mi caso para asegurarme el mejor acuerdo posible. Como un estudiante universitario que nunca ha hecho esto antes, explicaron los pasos a fondo y fueron muy comunicativos durante todo el proceso.
-Jared Wilson
Me gustaría dar las gracias al Sr. Peyton por asegurarse de que todo se explicó a fondo y también por ser uno de los mejores abogados que he tenido. Su personal fue muy amable y también se comunicó todo bien conmigo. ¡Yo recomendaría su bufete de abogados a todos mis amigos y miembros de la familia!
-Latrice White
Peyton trabaja duro para asegurarse de que los clientes están satisfechos. Fui herido en un accidente de coche hace dos años que dejó retos significativos. Él se aseguró de que yo fuera compensado justamente además de pagar todas las facturas médicas. Peyton es duro y trabajador, pero paciente y considerado con los clientes. Yo recomendaría Murphy...
-Brandy Williams
¡Murphy y su equipo son fenomenales! No tuve que preocuparme de nada. Se encargaron de todo por mí. Son muy profesionales y en cualquier momento que los necesites están ahí. ¡Este bufete de abogados se hizo cargo de mí!
-Na'Kiya Antonio
Fue una experiencia muy buena, todo el mundo era tan amable y servicial. Me encanta Murphy Law Firm.
-Janice Brown
No confío fácilmente en la gente. Desde el momento en que estreché la mano de Peyton Murphy supe que había algo diferente en él. Con preocupación y compasión se aseguró de que todas mis necesidades médicas fueran atendidas en primer lugar. Luego se aseguró de que yo estaba bien compensado por todos mis problemas y perdido. Yo...
-PRÍNCIPE JRDSQAUD
Desde el momento en que entré en la oficina, sentí que estaba en buenas manos. Peyton me aseguró que mi caso sería manejado correctamente, y Brian se aseguró de eso. Tengo y seguiré recomendando Murphy Law Firm a todos mis amigos y familiares.
-Jasmione Clark
Traté con la firma Peyton durante 5 años. Él representó a mi mamá, amigo niño grande y hermano. Peyton incluso envió flores para el funeral de los chicos grandes. Todavía tenemos las flores. sí bufete de abogados de buena calidad que le dan el mejor servicio y hacen el mejor trabajo Brian es el más maravilloso buen abogado de corazón ...
-Jeremy Douglas
Sr. Brian Mccullough nos ayudó y él está haciendo un gran trabajo. Yo lo recomendaría para cualquier persona que está buscando un abogado.
-Dulith Gurdiwasam Punchihewage
Al principio, estaba con un bufete de abogados diferente, uno al que siempre había recurrido antes. Con este caso, surgieron problemas, y decidimos separarnos - y entonces encontré a Peyton Murphy y su equipo. Sólo puedo decir que esta fue LA MEJOR decisión que tomé con respecto a mis asuntos legales. Este caso no fue...
-Hélice Tripal
Muy profesional, no tengo ninguna queja. Peyton Murphy fue muy servicial e ingenioso. Cualquier cosa que necesitaba, él y sus asociados estaban allí para ayudar día y noche. Hay un toque personal a la profesionalidad que él y su empresa ha mostrado. Recomiendo encarecidamente el uso de su empresa para sus necesidades legales. Gracias de nuevo.
-Justin Edwards
Yo estaba muy contento con Murphy Law Firm. Mi abogado, Jordan Gremillion - Yo estaba muy satisfecho con él, él me mantuvo actualizado con mi caso hasta el final. Él es un gran abogado y consiguió el acuerdo que quería, yo estaba contento con él y Betty. Si alguna vez necesito un abogado de nuevo,...
-Riptracks "theprinceofbatonrouge" ent
El bufete Murphy hizo un trabajo excelente. Peyton te hace sentir como si fueras viejos amigos. Los abogados que trabajan allí te hacen sentir muy cómodo. Denise, en particular, trabajó en mi caso. Esta fue la primera vez que necesitaba un abogado de lesiones y estoy muy feliz de haber elegido Murphy ...
-Mary
Todas las personas que conocí en esta empresa eran profesionales, amables, serviciales y estaban bien informadas. Estoy seguro de que la gente puede ver los anuncios y pensar que la preocupación es fingida, pero no lo es. Me trataron genuinamente con compasión y preocupación por mi bienestar. Junto con Peyton Murphy, Denise Bonnette y Brian McCullough fueron asignados como mis contactos principales ...
-Nicole Hazard
Murphy Law Firm hizo un trabajo impresionante para mí. Lucharon duro y nunca se rindieron. Estoy muy agradecido por todo lo que la Firma hizo por mí. Gracias de nuevo por todo su duro trabajo en mi nombre.
-Gloria Louis
Quiero AGRADECER al bufete de abogados de Murphy. Esta es mi segunda vez con esta firma. Sr. Peyton y su personal VA POR ENCIMA Y MÁS ALLÁ para usted. Agradezco profundamente a la Sra. Denise, que ANGEL. Le agradezco profundamente a la Sra. Denise, que es un ÁNGEL, y también al mismo Peyton, que es un ABOGADO INCREÍBLE, PERSONA. SU PERSONAL ES...
-Sharon Smith
Me recomendaron este bufete de abogados de un pariente. Mis interacciones con el Peyton Murphy, sus asociados, y el personal han sido una experiencia maravillosa teniendo en cuenta la situación. Me sentí informado y pude concentrarme en mi recuperación, sabiendo que mis asuntos estaban siendo manejados correctamente. Los resultados finales fueron mucho mejor de lo que me dijeron ....
-D__
Brian y Brooke son los mejores. Pude centrarme en la curación y en mi familia, mientras ellos se encargaban del resto. El resultado fue mejor de lo que esperaba. Si alguna vez necesito algo en el futuro, sé que voy a llegar a, y recomendar a todo el mundo.
-Angela Shields
El Sr. Murphy es un gran abogado. Él va más allá de sus clientes. Es muy atento a las necesidades de sus clientes. Por no hablar de que tiene un gran equipo que trabaja con él. ¡Gracias chicos por hacer un trabajo excepcional! Recomiendo encarecidamente Murphy Law Firm para hacer el trabajo.
-Tymesha "Ty" Plain
Estoy muy satisfecho con este bufete de abogados y todo su personal. Brian McCullough fue mi abogado y me trataron con la mayor profesionalidad y cuidado. Él está muy bien informado y fue informativo durante todo el proceso. Su experiencia es muy evidente. La experiencia y el profesionalismo que me encontré es, con mucho, por encima y...
-Sonya G
Peyton y Denise se hizo cargo de absolutamente todo después de mi accidente. De alquiler de coches a los especialistas médicos, tomaron lo que fue un momento muy estresante para mí, y lo hizo a donde yo tenía casi nada de qué preocuparse. Me mantuvieron plenamente informado durante todo el proceso, lo que condujo a un resultado muy positivo....
-Graham Hunt
Yo estaba muy contento con el servicio que recibí en la Firma de Abogados Murphy. Brian McCullough trabajó mi caso y sentí que fue más allá para proporcionar el mejor resultado para mi situación. La Sra. Betty llamaría y proporcionar una gran visión de cómo funciona el proceso y fue muy informativo sobre las actualizaciones...
-bdon
Resultados de los casos
Consulta gratuita
Iremos a verle
Orgullosamente al servicio del estado de Louisiana durante casi 30 años
Los campos marcados con un "*" son obligatorios

No se conforme
Para ¡Céntimos!
Baton Rouge Localización
2354 S Acadian Thruway
Baton Rouge, LA 70808 Cómo llegar
Nueva Orleans Ubicación
607 St. Charles Ave. Ste 300
Nueva Orleans, LA 70130 Cómo llegar
Teléfono:
Correo electrónico:
Horario:
De lunes a viernes
7.30 - 17.30 H
Derechos de autor © 2026 Murphy Law Firm. Todos los derechos reservados.

